In vitro Dermal Permeation Testing (IVPT) and Skin Absorption with ex vivo Skin and Mucosa, OECD 428, MDR 2017(745)
In vitro Dermal Permeation Testing (IVPT) and Skin Absorption – ex vivo Human and Porcine Skin, Buccal Mucosa, Nail Sheet
Chemicals, Agrochemicals, Cosmetics, Pharmaceuticals for Topical Application:
The assessment of percutaneous permeation of molecules is important for many products, such as dermal or transdermal delivery systems (pharmaceuticals, cosmetics), agrochemicals (pesticides, herbicides or fungicides). In vivo assessment for cosmetic products is not allowed in the EC since 2013 according to EU Cosmetic Regulation 1223/2009. For cosmetic compounds and drugs in the early phases of development alternative models have been developed, such as ex vivo human skin, usually obtained from plastic surgery patients, ex vivo animal skin or mucosa’s from slaughterhouse. The in vitro permeation test has been shown to be an ideal bio-equivalence model, it is accepted by authorities in OECD TG428 and in MDR EU Regulation 2017(745) for substance based medical devices. The assessment of percutaneous absorption or skin permeation is important for the successful development of new formulations intended for human use. The absorption model with animal buccal mucosa has become an attractive model for studies of local and systemic drug delivery, buccal mucoas serving also as vaginal model. Bovine nail sheets models have been developed for studies on nails.
Substance Based Medical Devices
The in vitro dermal permeation test (IVPT) allows also to risk assess the distribution of ingredients of substance based medical devices, a recent requirement by EU Regulation 2017(745), Appendix VIII, Rule 21. Substance-based medical devices are for example nasal/throat drops or sprays, syrups, vaginal creams or gels, skin creams for wounds, scars, etc. The IVPT is also applied for the classification of substance-based medical devices according to MDR 2017(745):
- Class III for systemic absorption of the product (metabolized or not) by the human body to achieve the intended purpose, or absorption in the stomach/lower gastrointestinal tract to achieve the intended purpose
- Class IIa for absorption and intended purpose on skin, nasal or oral cavity, including pharynx
- Class IIb for all other cases
We are pleased to represent these ex vivo skin models which are replacing animal testing and are fully in line with important 3R criteria: Replace, Reduce and Refine animal use in safety and efficacy evaluations.
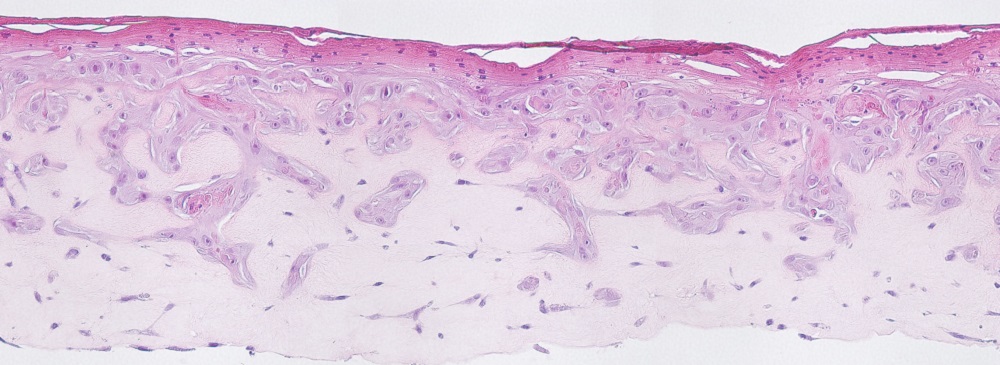
There are different ex vivo skin models available, such as full thickness skins, dermatomic sections or mucosas. The skins derive from different sources like abdominal or facial skin and are used for in vitro skin permeation and absorption studies according to OECD 428 or MDR 2017/745. The XenoSkin ex vivo human skins are prepared under high quality standards within up to 12 hours after cosmetic surgery for the in vitro use. Skin samples are collected from donors who gave their informed consent.
Characteristic Applications and Services available for in vitro Dermal Permeation Testing (IVPT)
- Regulatory studies in GLP according to OECD TG 428 EFSA, EMA and FDA guidelines
- Investigation of local distribution at application site
- Bio-equivalence studies
- Characterization and regulatory classification of Medical Devices according to Rule 21 in Appendix VIII of MDR EU Regulation 2017(745)
- Drug Delivery and Controlled Release Projects
- Absorption, Distribution, Metabolism and Excretion (ADME)
- Metabolites toxicokinetic on fully viable tissues
- HPLC-MS/MS, UPLC analytical techniques without radiolabelling
- Test compounds: chemicals, agrochemicals, cosmetics, pharmaceuticals (API), substance based medical devices
Ex vivo human skins of Xenometrix derive from abdominal or facial skins which are quality controlled for HCV, HIV. The average donor is 45 years with a skin thickness found in the average population.
- Human Abdominal Ex vivo Skin - Dermatomed Skin
- Human Abdominal Ex vivo Skin - Full Thickness
- Porcine ex vivo Ear Skin – Dermatomed
- Porcine ex vivo Ear Skin - Full Thickness
- Porcine Ex vivo Mucosa
- Bovine Ex vivo Nail Sheets

Animal Models
Ex vivo porcine ear skin models are also available for animal skin studies. Full thickness skins as well as dermatomic sections are offered. The XenoSkin ex vivo porcine skins are prepared under high quality standards. Buccal Mucosa is used also for different permeation models such as for colon or vaginal models. Bovine nails are used for studies on nails.
Franz Cells
The Franz Diffusion Cells include an ex vivo human or animal skin and imitates the behaviour of chemicals, cosmetics, pharmaceuticals when applied to skin. Ex vivo skin discs are fixed in glass Franz Diffusion Cells between acceptor and receptor compartment and the test item is added above the skin disc, membrane respectively. The rate of transfer is determined by collection of the permeate in the sampling device of the Franz Cell. Usually, a phosphate buffer is added as receiving solution. The liquid in the receptor cells is analysed by HPLC. Franz Cells can be jacketed with controlled temperature water flow.
Jacketed and Unjacketed Franz Diffusion Cells (FDC) of many different sizes are available.
FDC 5-2.5: Diameter 5 mm, surface 0.20 cm, acceptor volume 2,5 ml
FDC 7-2.5: Diameter 7 mm, surface 0.38 cm, acceptor volume 2,5 ml
FDC 9-2.5: Diameter 9 mm, surface 0.64 cm, acceptor volume 2,5 ml
FDC 4-12: Diameter 4 mm, surface 1.00 cm2, acceptor volume 12 ml
FDC 15-12: Diameter 15 mm, surface 1.76 cm2, acceptor volume 12 ml
FDC 20-10: Diameter 20 mm, surface 3.14 cm2, acceptor volume 10 ml
FDC 20-15: Diameter 20 mm, surface 3.14 cm2, acceptor volume 15 ml
FDC 2-20: Diameter 2 mm, surface 4.98 cm2, acceptor volume 20 ml
FDC 8-120: Diameter 8 mm, surface 6.51 cm2, acceptor volume 120 ml


 Navigation
Navigation