Ames Test - Scientific Background
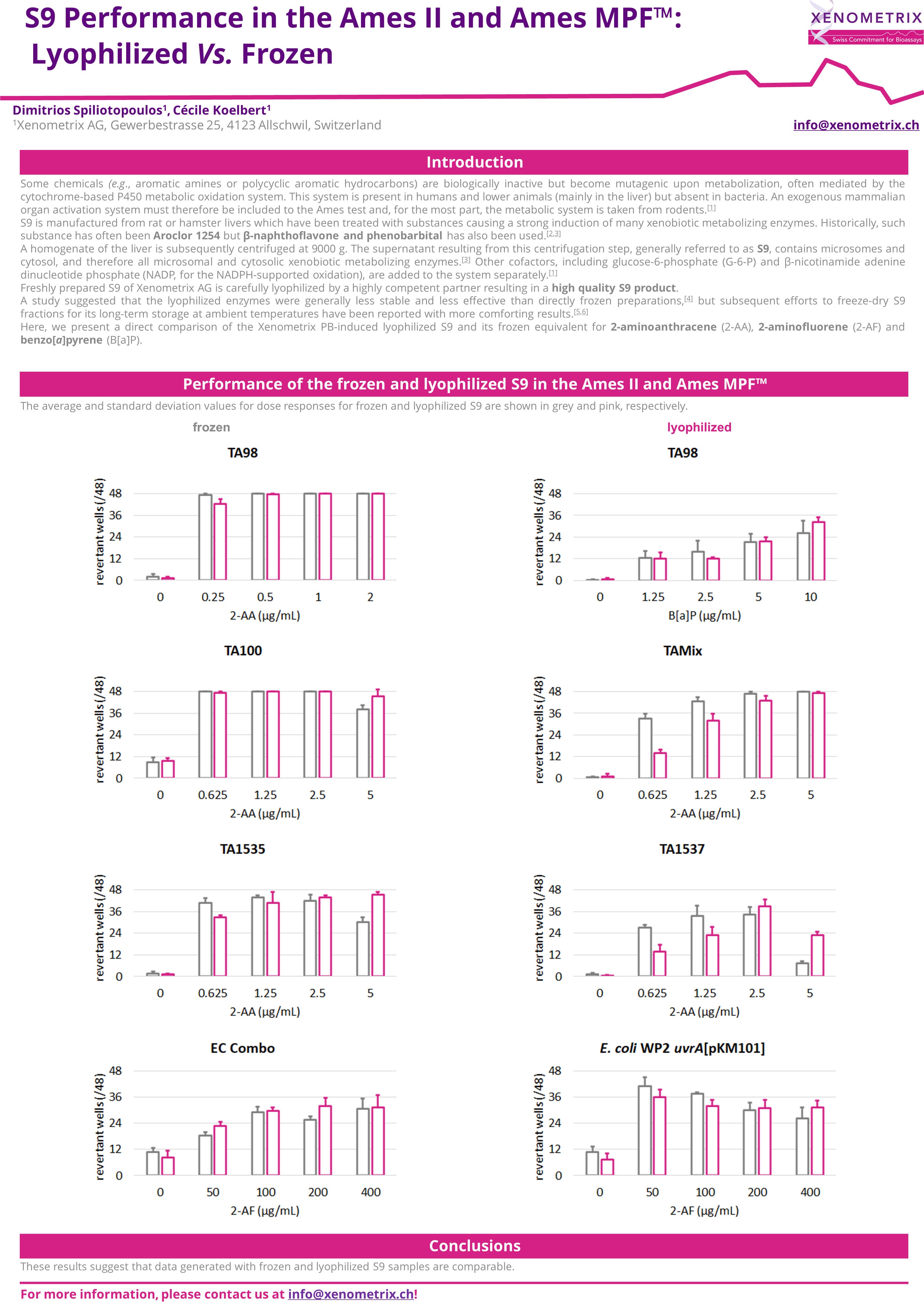
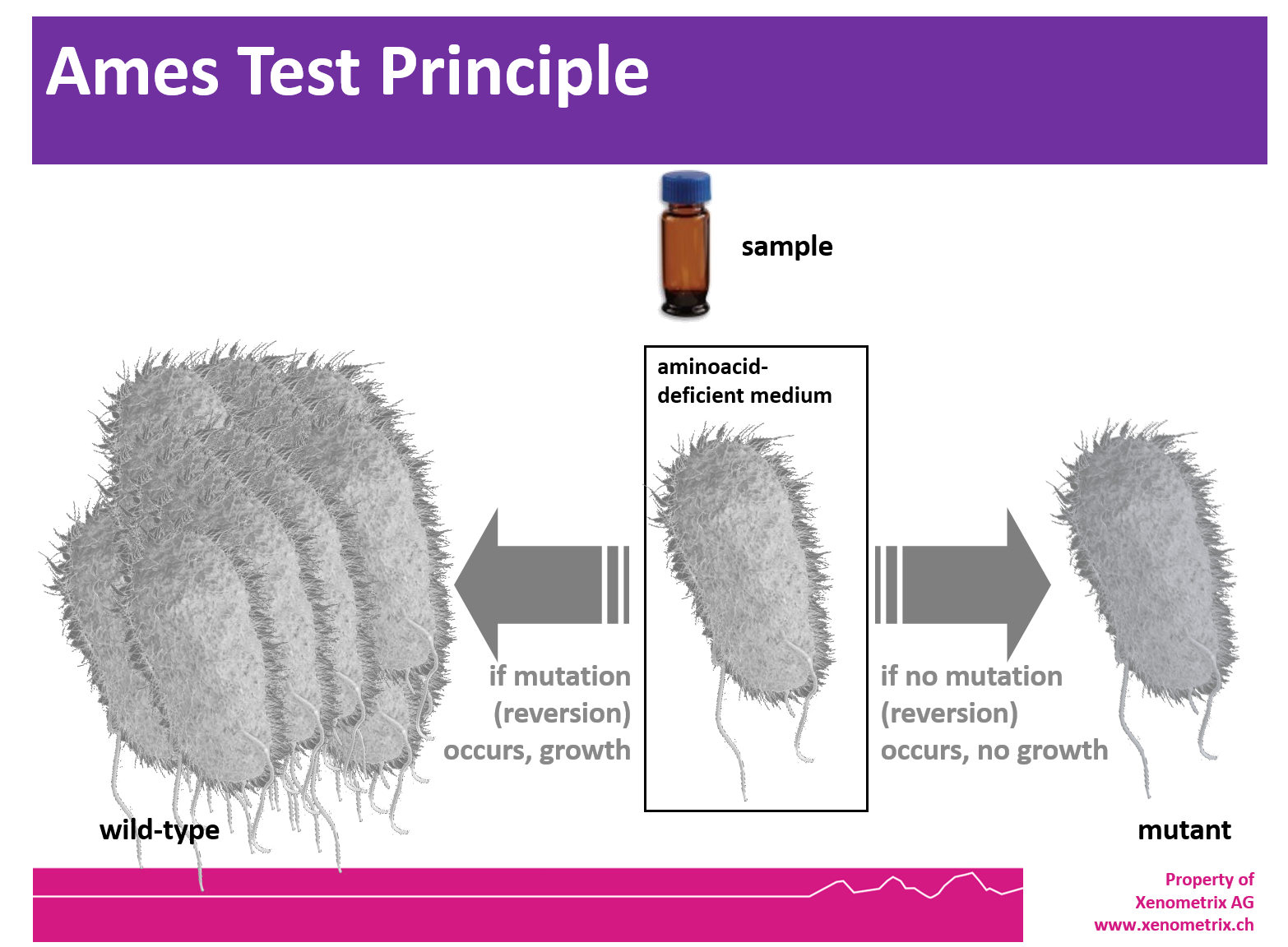
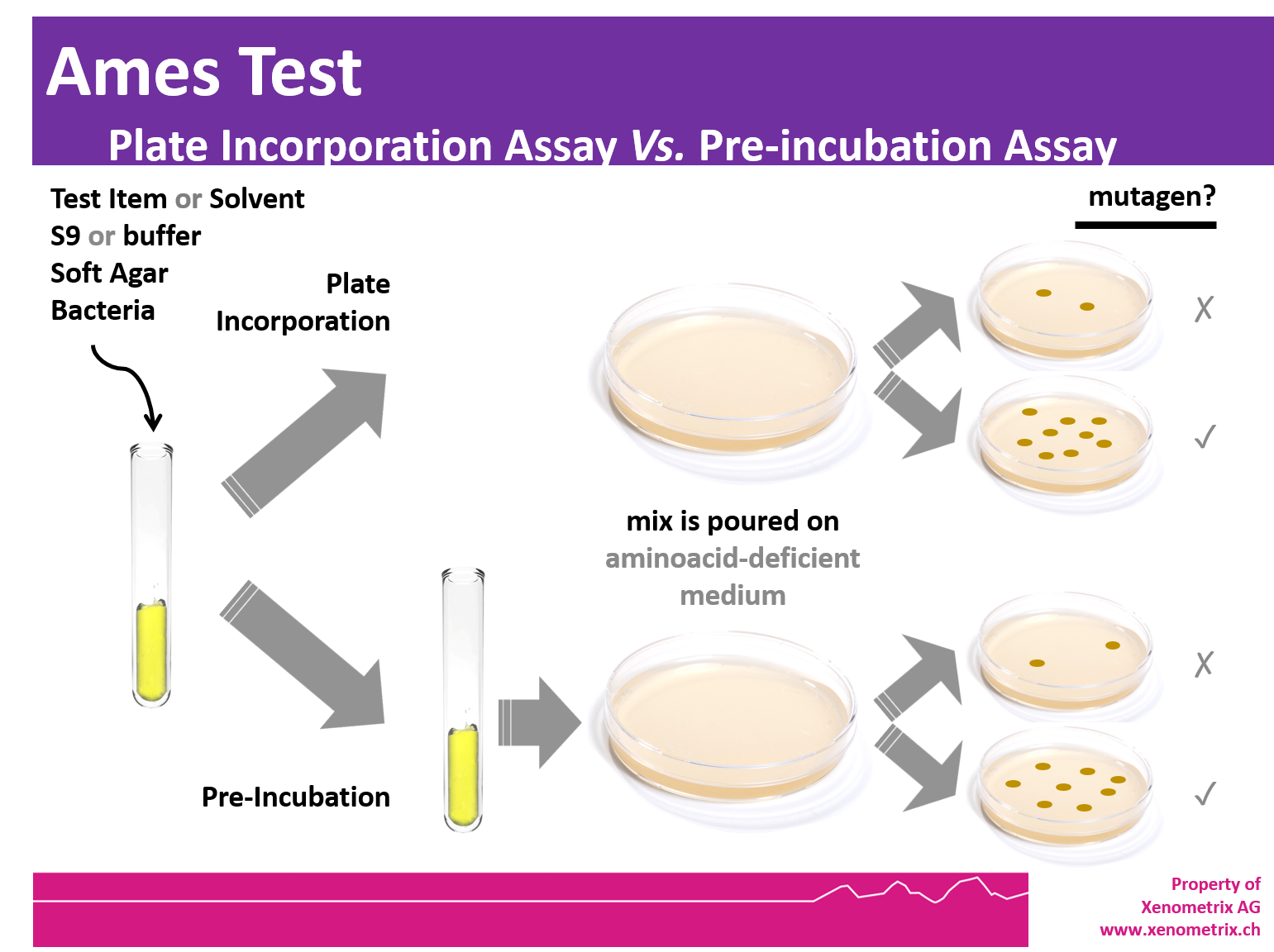
Ames Test - Principle
The Ames tester strains are S. typhimurium and E. coli strains which have been used for more than 40 years to detect mutagenic compounds. Point mutations were made in the histidine (Salmonella typhimurium) or the tryptophan (Escherichia coli) operon, rendering the bacteria incapable of producing the corresponding amino acid. These mutations result in his- or trp- organisms that cannot grow unless histidine or tryptophan is supplied.
A test sample's mutagenic potential is assessed by exposing these amino acid-requiring organisms to varying concentrations of sample and selecting for the reversion event. Media lacking histidine or tryptophan are used for this selection which allow only those cells that have undergone the reversion to histidine / tryptophan prototrophy to survive and grow. A mutagenic event causing base substitutions or frameshift mutations within the gene may cause a reversion to amino acid prototrophy. These reverted bacteria will then grow in histidine- or tryptophan-deficient media, respectively, whereas non reverted bacteria will not be able to grow. The media during the growth phase can be liquid or agar based.
Ames Test on Agar Plate OECD 471 – Assay Description
Freshly thawed frozen strains are inoculated in growth medium and the cultures are grown overnight at 37°C in an environmental shaker in the presence (TA98, TA100, E. coli WP2 [pKM101], E. coli WP2 uvrA[pKM101]) or absence (TA1535, TA1537, E. coli WP2 uvrA) of ampicillin. The overnight cultures are used the next morning for the Ames plate-incorporation or pre-incubation assay.
The tester strains are exposed to the chemical for 20 min at 37°C prior to plating on minimal glucose agar plates. The components of the preincubation mixture are S9 mix or phosphate buffer, test chemical solution and bacterial culture. After 20 min at 37°C, molten top agar supplemented with low histidine/biotin or tryptophan and maintained at 48°C is added to the mixture. The contents are mixed and poured onto minimal glucose agar plates. After 48 – 72 hours at 37°C, the number of colonies per plate and per dose is counted by eye and compared with the number of spontaneous revertant colonies obtained in the negative control plates. Guidelines addressing the Ames Test are OECD 471, ICH M7.
MicroAmes24 - Miniaturized 24-well agar plate Ames Test
Liquid Microplate Fluctuation Ames Test OECD 471, ICH M7 – Assay Description
Overnight growth is identical to the procedure described before (Ames Test on Agar Plates – Assay Description).
Bacteria are exposed to 6 concentrations of a test sample, as well as a positive and a negative control, for 90 minutes in medium containing enough histidine (S. typhimurium) or tryptophan (E. coli) to support approximately two cell divisions. After exposure, the cultures in each condition (negative control, test samples and positive controls) are diluted in pH indicator medium lacking histidine or tryptophan and aliquoted into 48 wells of a 384-well plate.
Within two days, cells that have undergone reversion to amino acid prototrophy will grow. In the liquid Ames II/Ames MPF™ system, bacterial metabolism reduces the pH of the medium, changing the colour of the well the bacteria are in. The number of wells containing revertant colonies are counted for each dose and compared to a solvent (negative) control. Each dose is tested in triplicate to allow for statistical analysis of the data.
A dose-dependent and significant increase in the number of revertant colonies upon exposure to test sample relative to the solvent controls indicates that the sample is mutagenic.
The mutagenic potential of samples is assessed directly and in the presence of metabolic activation, provided by a liver homogenate, S9.
Guidelines addressing the Ames Test: OECD 471, ICH M7.
Ames MPF™ Test step-by-step video
This video shows how to run the Ames MPF™ or the Ames II assay step by step helping you to set up the assay. It also shows all the instruments and material required to run the Ames MPF™ or Ames II assay.
Ready-to-Use Kits or Individual Products?
Ready to use test kits including strains, media, ampicillin, S9, positive controls are available in the liquid microplate format Ames MPF™ as well as in the 6 or 24 well agar plate format. The ready to use kits are quality controlled by phenotype of the strains, by positive controls as well as by rat liver microsomal fraction S9 and therefore are a more standardized system. Stability studies, growth control, toxicity studies of S9 and positive controls are continuously performed.
More Information:
Read here a Stability Study of Ames Tester Strains
Available Ames MPF™ Kit Systems, Liquid Microfluctuation Assay:
- Ames MPF™ Penta 1
- Ames MPF™ Penta 2
- Ames MPF™ 98/100
- Ames II
- Ames MPF™ Aqua for environmental samples according to ISO11350
Available Ames Agar Plate Kit Systems:
- MicroAmes24
- NanoAmes™ (in development)
Ames Test Strains – Genotypes
E. coli Ames tester strains as well as S. typhimurium Ames tester strains have been used for more than 40 years to detect mutagenic compounds in chemicals, pharmaceuticals, cosmetics, biocides, water and other environmental samples. They are all listed in the guideline OECD 471: Bacterial Reverse Mutation Test and in guideline ICH M7 for genotoxic impurities. Point mutations were made in the histidine (Salmonella typhimurium) or the tryptophan (Escherichia coli) operon, rendering the bacteria incapable of producing the corresponding amino acid. These mutations result in his- or trp- organisms that cannot grow unless histidine or tryptophan is supplied.
A test sample's mutagenic potential is assessed by exposing these amino acid-requiring organisms to varying concentrations of sample and selecting for the reversion event. Media lacking histidine or tryptophan are used for this selection which allow only those cells that have undergone the reversion to histidine / tryptophan prototrophy to survive and grow. A mutagenic event causing base substitutions or frameshift mutations within the gene may cause a reversion to amino acid prototrophy. These reverted bacteria will then grow in histidine- or tryptophan-deficient media, respectively, whereas non reverted bacteria will not be able to grow. The media during the growth phase can be liquid or agar based
E.coli WP2 pKM[101] strains are used in agar based Ames Test, in miniaturized agar based (MicroAmes24) and liquid format Ames Test (Ames MPF™). The strains are used in accordance with Guidelines OECD471, ICH M7.

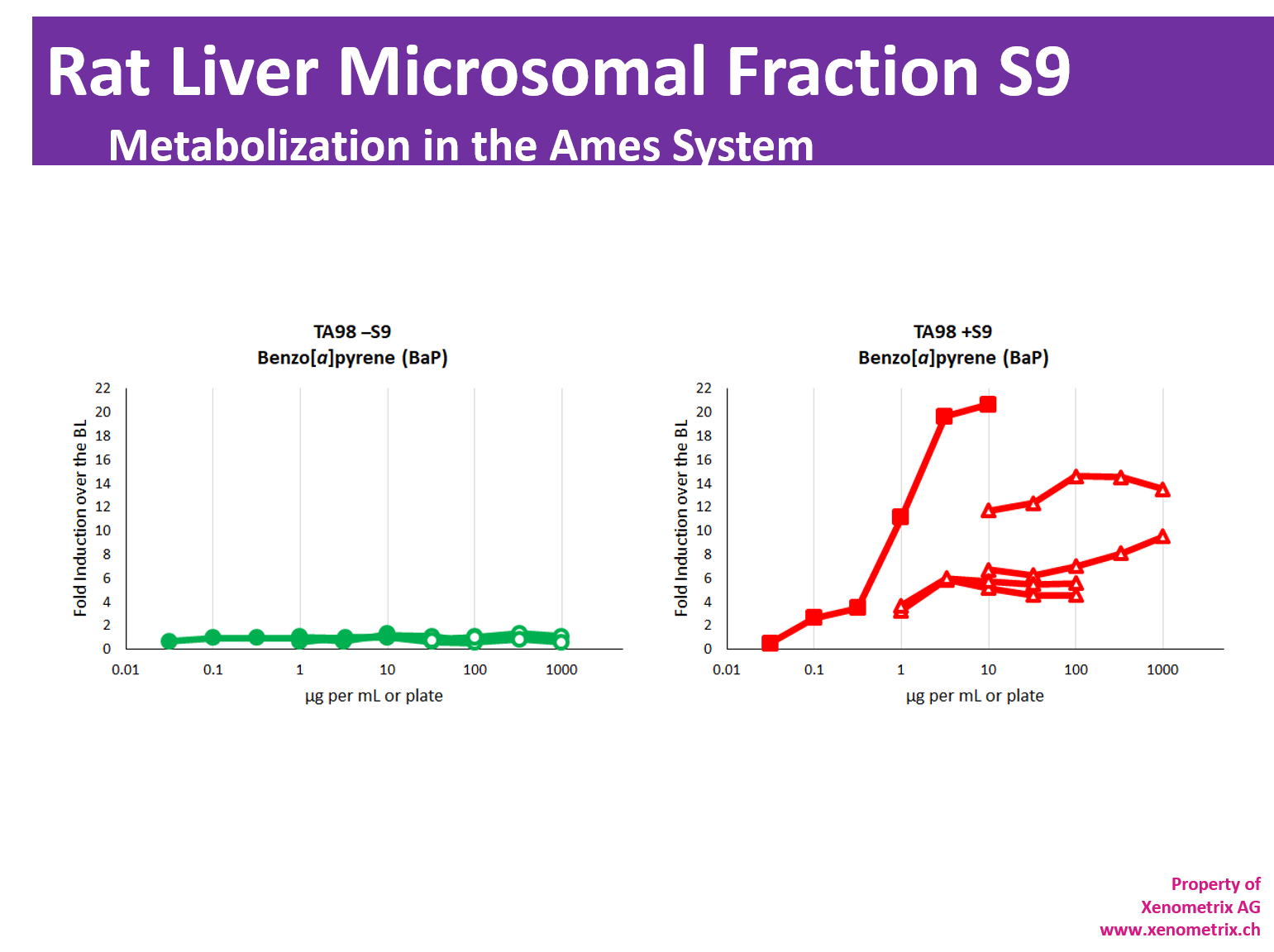
Metabolization in the Ames test – Rat Liver Microsomal Fraction S9
The majority of molecules develop their genotoxic activity only after metabolization. Due to bacteria being unable to metabolize molecules, the mutagenic potential of samples is assessed directly and in the presence of metabolic activation system: S9 Rat liver microsomal fraction S9 fraction of rat liver homogenate is obtained after the first centrifugation step at about 9000×g and is mimicking liver metabolism in in vitro test systems. The liver S9 fractions contain both microsomal and cytosolic fractions. They include phase I and phase II enzymes and cofactors.
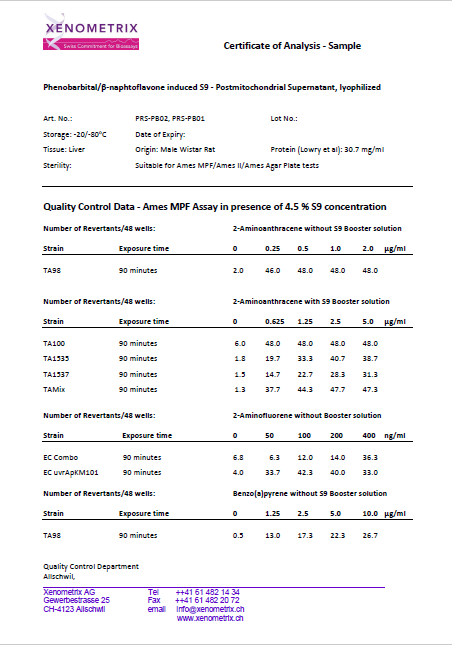
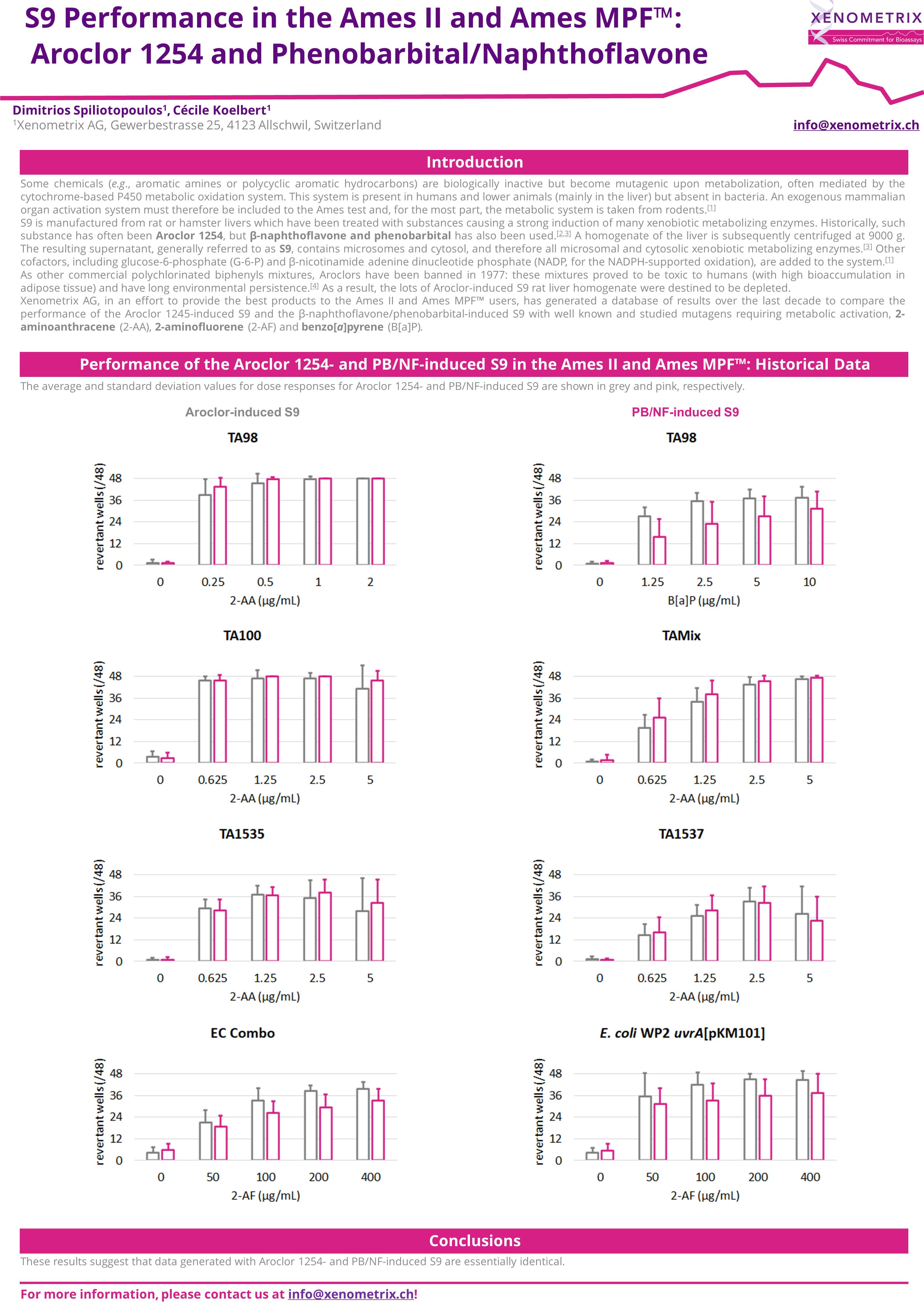
Expression of liver enzymes has been historically induced with Aroclor 1254 or, more recently, Phenobarbital/β-Naphtoflavone.
It is worth mentioning that Aroclor 1254 is in conflict with Stokholm Convention on Persistent Organic Pollutants and has been banned. Therefore, the availability of the Aroclor 1254-induced S9 cannot be guaranteed in the future.
Metabolization of test compounds must be taken into account in all genotoxic studies and environmental safety assessments of chemicals according to OECD TG471, ICH M7 or in vitro mammalian cell micronucleus test OECD TG487
Comparison of the Aroclor 1254- and Phenobarbital/Naphthoflavone-induced S9 in the Ames II/Ames MPF™
Comparison of the frozen and lyophilized S9 in the Ames II/Ames MPF™
Automation of the Ames MPF™ Test
- Pipeting robot transfers the liquids from 96-well sample plate to 24-well and 384-well plates
- Ames MPF™ Test kits are most convenient model for automation
- Benefits: Reduction of hands on time, test compound quantity, S9, plastic waste
- Metafer (metasystems-international.com) allows automated reading and archiving of Ames MPF™/Ames II, micronucleus or chromosomal aberration test results
- Metafer scans specimen of various sizes, uses many different contrasting methods and magnifications
- Modularity of Metafer allows several applications such as cytogenetics, toxicology, pathology, haematology.

umuC Easy Test - SOS Response Assay
Xenometrix offers the umuC Easy kit, a procedure based upon the International Organization for Standardization protocol ISO 13829 (Water Quality-Determination of the genotoxicity of water and waste water using the umu-test).
umuC Test Principle
The umuC Easy test kit is based on a genetically engineered Salmonella typhimurium TA1535 [pSK1002] which measures the SOS response of a cell to genetic damage. In just 16 hours, the kit provides a clear, quantitative measurement of the genotoxicity of a sample by simple colorimetric detection.
Assay Description
Salmonella typhimurium TA1535 [pSK1002] bacteria are exposed to potentially genotoxic test compounds. If genotoxic lesions are produced by the test compound, the umuC gene is induced as part of the bacterial SOS response. The induction of lacZ on the the plasmid pSK1002 is measured by the conversion of colorless ONPG substrate (o-nitrophenyl-β-D-galactopyranoside) to the yellow product o-nitrophenol by the lacZ-encoded β-galactosidase.
Since different kinds of genotoxic lesions lead to the induction of the SOS response, one strain of S. typhimurium with the appropriate reporter gene construct is sufficient to identify all classes of bacterial genotoxins. As with other bacterial genotoxicity and mutagenicity assays, compounds requiring metabolic activation for activity can be identified by the addition of S9 microsomal rat liver extract.
The protocol has been adapted from ISO 13829 "Water quality - Determination of the genotoxicity of water and waste water using the umu-test". All media and reagents except for the S9 co-factor concentrations are as described in ISO 13829 and can be used with the original ISO 13829 protocol or with this optimized umuC- Easy CS protocol.
Ames Test – References
- Ames BN, Lee FD, Durston WE. 1973. An Improved Bacterial Test System for the Detection and Classification of Mutagens and Carcinogens. PNAS USA. 70(3):782–6.
- Ames BN, Durston WE, Yamasaki E, Lee FD. 1973. Carcinogens are Mutagens: A Simple Test System Combining Liver Homogenates for Activation and Bacteria for Detection. PNAS USA. 70(8):2281–5.
- McCann J, Choi E, Yamasaki E, Ames BN. 1975. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. PNAS USA. 72(12):5135–9.
- Ames BN, McCann J, Yamasaki E. 1975. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 31(6):347–63.
- Mortelmans K, Zeiger E. 2000. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 455(1–2):29–60.

 Navigation
Navigation