
UmuC
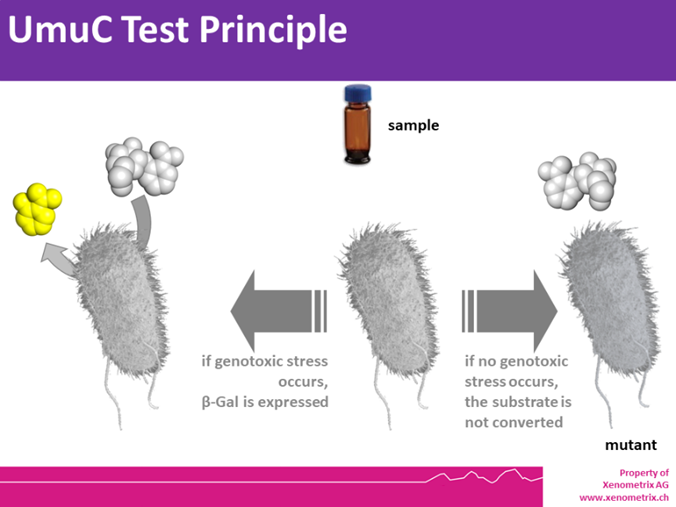
UmuC – Principle
The SOS/umu test was developed in an effort to test water samples in an efficient and cost-effective manner. Originally devised to be run in test tubes, the assay was adapted to microplate and expanded to other types of samples (e.g., chemicals, environmental samples and pharmaceuticals).
The SOS regulatory system is maintained in the repressed state by the protein LexA. Upon DNA damage (due to radiation or chemicals), RecA is converted into a protease that acts on LexA, thereby derepressing (activating) the SOS system, which in turn activates a number DNA repair genes, including those of the umu operon.
The method was originally developed for environmental monitoring, in particular to detect genotoxicity of water and waste water.
UmuC Tester Strain – Genotype
The tester strain is S. typhimurium TA1535 with the plasmid pSK1002, which carries a fused umuC’–‘lacZ gene . In this strain, lacZ is up-regulated as a result of the activation of the umu operon, allowing to monitor spectrophotometrically the β-galactosidase activity and hence the extent of DNA damage.
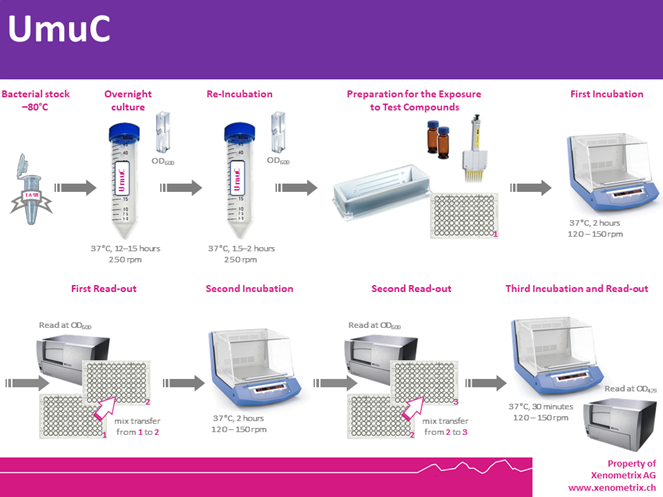
UmuC – Assay Description
Bacteria are exposed to different and low concentrations of a test sample in 96-well microtiter plates, as well as a positive and a negative control. Subsequent incubations and spectrophotometric readings allow to quantify the activation of the umuC promoter within a 2-3 hours.
A formula allows to calculate β-galactosidase activity and percentage survival. A dose-dependent and significant increase in the expression of β-galactosidase over the background (associated with adequate survival) indicates that the sample led to DNA stress.
The mutagenic potential of samples is assessed directly and in the presence of metabolic activation, provided by a liver homogenate, a post-mitochondrial supernatant fraction S9, respectively, essentially in the same way as in the Ames test.
Ready-to-Use Kits or Individual Products?
Ready to use test kits including strain, media, ampicillin, S9, positive controls are available in the liquid microplate format UmuC Easy CS for the 96-well plates. The ready to use kits are quality controlled by phenotype of the strains, by positive controls as well as by rat liver microsomal fraction S9 and therefore are a more standardized system. Stability studies, growth control, toxicity studies of S9 and positive controls are continuously performed.
The products can also be used with an HPTLC (high-performance thin-layer chromatography) approach for an improved sensitivity and/or to allow subsequent biological or physical analyses.
Individual items of the UmuC Easy CS kit system are available as well.
UmuC – References
- Oda Y, Nakamura S, Oki I, Kato T, Shinagawa H. 1985. Evaluation of the new system (umu- test) for the detection of environmental mutagens and carginogens. Mut Res. 147(5):219–29.
- Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K. 1987. SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: Examination with 151 chemicals. Mut Res. 192(4):239–46.
- Reifferscheid G, Heil J, Oda Y, Zahn RK. 1991. A microtitre version of the SOS/umu-test for rapid detection of genotoxins and genotoxic potentials of environmental samples. Mut Res. 253(3):215–22.
- Reifferscheid G, Heil J. 1996. Validation of the SOS/umu test using test results of 486 chemicals and comparison with the Ames test and carcinogenicity data. Mut Res. 369(3–4):129–45.
- Meyer D, Marin-Kuan M, Debon E, Serrant P, Cottet-Fontannaz C, Schilter B, Morlock GE. 2020. Detection of low levels of genotoxic compounds in food contact materials using an alternative HPTLC-SOS-Umu-C assay. ALTEX. 38(3):387–97.

 Navigation
Navigation